|
|
|
Electron Orbit Question and Answer Forum
Q17: October 20, 2003: I do not understand why in your mind there is something more fundamental with the electron and consider it a particle that exists, but a photon does not. You can perform the double slit experiment with an electron or photon and obtain the same wave / particle issue depending on the experiment.
A17: You are able to "catch" an electron, hold it still and observe it. You can not do the same with a photon. It is this characteristic of being able to observe an entity at rest that makes it substantive. It seems to appear as well that, when an entity can be observed in this way, it also has mass and occupies a regionally distinct volume. Now when we discuss a wave, we never hold this level of observation to be true. We understand that a wave is the sum of a multitude of positions, velocities, forces, vectors, etc. and to "catch" a wave, hold it still and observe it, is meaningless.
When the double slit experiment is conducted with light, it is fairly straight forward to understand the effects, if light is considered to be a wave being detected by electrons. When a single "photon" is "emitted", we may consider that a single electron in the source moved from the excited state to its rest state. This means that one and only one electron at the target capable of absorbing the discrete amount of energy will transition to the excited state. To excite more, would violate the Law of Conservation of Energy. To excite none, would also violate LCE. Energy can be moved in only discrete packets as permitted by the stable orbits of the atoms involved (we are discounting thermal losses due to energy spent shaking atoms around and energy lost moving conduction band electrons in metals). From this we can construct a wave that triggers only one of the two detectors. It is the probability that an electron is in the right position to absorb the momentum (and thus the energy) communicated by the wave that determines which detector signals the event.
Now, it would seem that electrons should not generate an interference pattern, for to do so implies a wave for constructive/destructive interference. There are two things most relevant to this experiment - the dimensions of the aperture through which the electron must pass and the presence of a neighboring aperture. The apertures must be longer than they are wide thus creating a pair of slotted "one dimensional" cavities. Though it is tempting to think that an encroaching electron just bounces off the "sides" of the aperture on its flight to the target, there is no reason to expect that this is the case. Indeed, there is every reason to expect the electron becomes trapped in this resonant cavity until subsequent electrons cause it to be ejected toward the target. During the short time the electron is "trapped" in the slot, it oscillates in the cavity and induces an electron in the neighboring cavity to oscillate as well. These oscillations set up a field between the apertures and the target which induce electrons to travel along paths of least resistance that result in the detected interference pattern. Placing detectors that disrupt this field or that interact with the electron during its flight disturb the resulting interference pattern.
Q16: August 18, 2003: In section 1.3 the Lorentz transformations are not correct. They are missing "-ut" for the x' tranformation and "-(u/c2)x" for the t' transformation.
A16: Indeed, the Lorentz transformation reduces to Einstein's postulates for x = t = 0. This in no way affects the outcome of the theory.
Q15: August 9, 2003: What does this theory reveal about the splitting of spectral lines, especially when the radiating atoms are exposed to magnetic and electric fields?
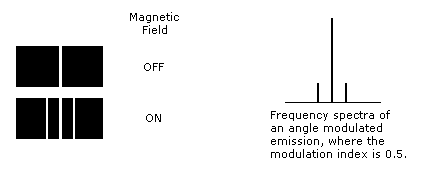
A15: In the table at the end of section 1.4, it is shown that multiple energy levels are associated with various values of "n". Answer 11 (below) proposes a mechanism for fine structure (closely spaced pairs of emission lines). In the simple case of a hydrogen atom, an electron in orbit around the nucleus would form a small magnetic dipole that is free to interact with external magnetic fields (normally this dipole is continuously moving in random directions). Applying an external magnetic field aligns the direction of the dipole and the electron orbit orientation (i.e. the axis around which the electron orbits will be aligned with the external magnetic field). When the spectra of excited atoms is viewed under these conditions an additional paired split in the spectra is observed (see figure) that shows greater spread as the magnetic field is increased (i.e. the Zeeman effect). This is not difficult to understand, if the external magnetic field, F, imposes an angle modulation component onto the fundamental emission frequency, we (that is, e(t) = cos(wet + b*sin(wFt))). As long as the modulation index, b, remains less than ~1 the spectra will split into a pair of emission lines around a central one (i.e. the majority of the power is in the fundamental and the two sidebands). However, with atoms capable of larger modulation indices, multiple emission lines appear (i.e. anomalous Zeeman effect). Further investigation would be necessary to determine how closely actual spectra match with this explanation. The Stark effect is thus the electric field equivalent (see The Feynman Lectures on Physics Volume II page 13-6).
Q14: July 1, 2003: In section 1.5 what you call the centripetal force is in fact the centrifugal force i.e. it pushes orbiting objects away from the center of revolution, countering the centripetal force, eg, electric attraction. The centripetal force tends to draw orbiting objects toward the center of revolution to maintain their orbital motion.
A14: You are correct and the change is reflected in the text. The centripetal force is directed inward so the electrostatic force is the source of the centripetal force, that is Fe = Fc and Fe - Fc = 0. If an observational reference frame is placed above the orbital axis looking "down" and rotated at the same velocity as the orbit, the two bodies appear at rest a fixed distance from each other. If Fe were to suddenly cease, the orbiting body would appear to be under the influence of a force pushing it outward in a straight line. In this scenario, we could describe Fe as the attractive (i.e. positive) electrostatic force and Fo a repulsive (i.e. negative) outward force equal to Fc.
Q13: August 8, 2002: In the previous answer, you say that "it follows that, increasing the intensity, will cause more electrons in the metal to attain sufficient momentum to be knocked free of the surface." It would seem that the opposite should occur - if there is a greater intensity, then the electron would attain a higher velocity.
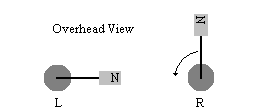
A13: Suppose we have a simple system shown in the following figure. It consists of two magnets, each attached to a rod that is free to rotate. Magnet R begins rotating counterclockwise, as indicated by the arrow, causing magnet L to be kicked into rotation at less than or equal to the velocity of magnet R. If the magnetic field from R is not too strong and there is friction on L, L will come to rest facing somewhere to the left. Now suppose magnet L is surrounded by many R magnets, all spinning with the same frequency and phase. What would the velocity of L be? Imagine ten L magnets surrounded by a sea of R magnets. Would nine of the L magnets be at rest and one spin at ten times the frequency (i.e. have 10x velocity)?
Q12: August 1, 2002: Referring to Q4, if light is not a photon (a particle that carries a scalar quantity - energy), how is it that increasing the intensity of the light of insufficient frequency does not cause electrons to be emitted from a metal surface? The classical wave theory would suggest that increasing the energy means increasing the intensity (i.e. amplitude) and, thus, the kinetic energy of electrons knocked free of the surface. Measurements do not support either of these wave theory conclusions.
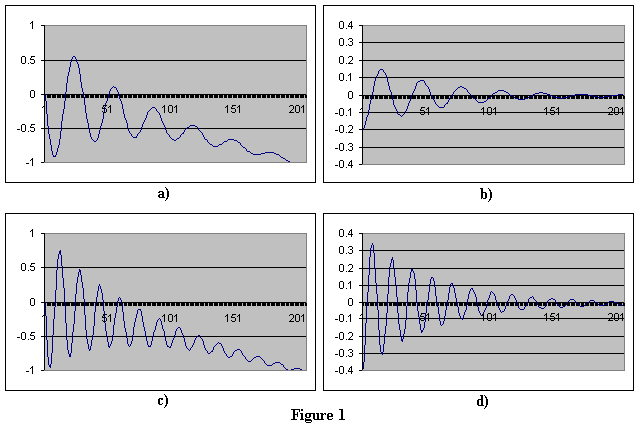
A12: Recall that equation 7 rests on the premise that discrete orbits exist where the momentum lost to space is zero. If this orbiting electron is able to exchange enough momentum (a discrete amount) with an outside partner(s) via the electric field, it has the potential to move to another stable orbit. Since momentum must be conserved, the partner loses or gains accordingly, otherwise no sustained exchange occurs. In addition, we must consider the frequency and phase associated with any movement between orbits, since this affects the total effective exchange of momentum. An electron in a stronger electric field (i.e. closer to the nucleus), has a higher resonant frequency than one further out. These three factors, amount of momentum, frequency and phase, combine to make a very selective environment for the exchange of momentum. Suppose we have an electron that undergoes the positional displacement shown in Figure 1a. The derivative (i.e. velocity) is shown in 1b. Additionally, suppose this electron undergoes another positional displacement, but with a higher frequency (Figures 1c & 1d). Here we see in Figures 1b and 1d that the momentum (mass * velocity) is greater for the higher frequency electron.
It is clear from this description that the amplitude of the momentum is related to the frequency and that the momentum, in order to remain conserved, must be transferred in discrete amounts. Now let me address each of your objections:
How is it that increasing the intensity of the light of insufficient frequency does not cause electrons to be emitted from a metal surface?
Since a wave of lower frequency communicates less momentum and its frequency is not conducive to an efficient exchange, it is unable to transfer what momentum it has to a potential electron. Increasing the intensity serves to increase the overall momentum (presuming it is in phase), but the frequency is such that the momentum can not be transferred effectively.
The classical wave theory would suggest that increasing the energy means increasing the intensity (i.e. amplitude) and, thus, the kinetic energy of electrons knocked free of the surface.
The amplitude of the wave I am discussing is the momentum, which is affected primarily by the frequency of the oscillating electron. The way the intensity is increased is by exciting more electrons into oscillation. It follows that, increasing the intensity, will cause more electrons in the metal to attain sufficient momentum to be knocked free of the surface. They will not necessarily attain greater kinetic energy.
Here is an objection that was not cited - The wave theory would suggest that the electron should absorb energy over an extended period until it has acquired enough to be emitted. No time lag, due to this effect, has ever been measured.
For an electron to "absorb" energy, it must attain a greater velocity and, thus, momentum. Discrete momentum levels are associated with the stable orbits, and any momentary change in momentum that is insufficient to attain another orbit, will be immediately "radiated" away.
I should also note, that since we are concerned about momentum as a vector quantity and these vector's directions may be randomly distributed, there is the possibility that, even though there may be sufficient momentum/frequency to cause an electron to shift orbits, it may not be in the proper phase to effect the exchange.
Q11: July 20, 2002: It appears that the value for a, just prior to equation 7, is very close to the value of the fine structure constant. It is also unitless, just like the fine structure constant. Are these the same quantities?
A11: It is conceivable that the fine structure constant could be inserted for the value of a in equation 7. This would change slightly, but to no significant degree, the radii and energy of the orbits. Recall that in my derivation, I used the atomic size of hydrogen to compute a. However, I am reluctant to make this substitution generally, and this is why. I have defined a as the average speed by which the momentum of the electron is exchanged with the proton. This means that for different atoms (i.e. different number of nucleons), there will be different values for a as the mass and field strength of the nucleus varies.
I should also draw your attention to the apparently "unused" orbits in the table that immediately follow equation 8. If you are familiar with the fine structure constant, you'll know that its origin came from a slight split found in the spectral signature of hydrogen. This was later attributed to the "spin" of the electron. Just the same, I'd like to think that it arises from a simpler underlying mechanism similar to the one shown below that arises naturally from the theory:
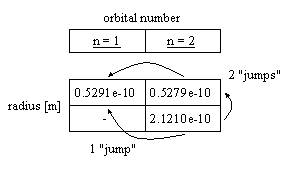
PS: After further consideration, I am prepared to answer "yes" to this question, that is, that a from equations 5 & 7 is the fine structure constant. However, it is a function of the number of protons (more specifically, the total net charge) in the nucleus.
Q10: March 1, 2002: Neutrinos do not have charge and rarely interact with matter.
A10: The neutrino does react with matter, albeit very rarely. For this interaction to be evident, the neutrino must have some intrinsic property in common with "regular" particles (see A5). If the charges are very close to each other, as in the case of the neutron and neutrino, the particle appears neutral in the far field. However, once in near field proximity to another charged particle, the interaction is evident.
Q9: February 26, 2002: There seems to be an obvious error in A1. The free neutron decays into a proton, electron and an antineutrino.
A9: Theory states that an "anti-neutrino" is released when the neutron decays. The difference between a neutrino and its "anti-" is the angular momentum along the direction of motion (i.e. spin). The neutron (spin +1/2) would then be comprised of a proton (+1/2), an electron (+1/2) and an "anti-"neutrino (-1/2). Since electrons may have +/-1/2 spin, I would suggest that this is not an intrinsic property of the neutrino either, but rather a result of an interaction.
Q8: January 27, 2002: The steps following equation 7 are difficult to follow. Can you be more specific?
A8: Here are the step by step operations:
e2/4pe0r2 = mse2/r -- equate the electrical and centripetal forces
e2/4pe0c2 = mse2r/c2 -- multiply both sides by r2/c2
e2/4pe0c2 = (me0/cosqe)(h2r) -- substitute h for se/c and express the mass in relativistic form
e2/4pe0me0c2 = (h2/cosqe)r -- get the constants to the left side
K = r (h2/cosqe) -- simplify by combining the constants into one single constant, K
where: K = e2/4pe0me0c2
K = r (sin2(qe)/cosqe) -- make substitution as indicated (reference Equation 2)
where: sinqe = h
K = r tanqesinqe -- substitute tan for sin/cos
r = K / tanqesinqe [m]
Q7: November 5, 2001: What you have described by your equation is an equilibrium point but not a stable equilibrium point. It is mearly a ledge on the wall of a cliff. Once the electron is disturbed, it will fall toward the nucleus.
A7: Instead of a cliff, let us visualize a bowl with a "ledge" part way up. The ledge is the zone where energy is not lost (for our example, it is frictionless). A marble introduced at the top of the bowl will spiral around the wall of the bowl losing heat energy due to friction as it does. When it gets to the level of the frictionless ledge, it will continue circling at this level. By definition (see text), the marble (electron) no longer dynamically interacts with the outside world (i.e. momentum lost to space is zero). This is not to say that the static charge is not conserved - it is. However, interactions will happen, and the marble, once knocked off the ledge, will have additional energy to go up the wall or be sent toward the bottom. In the latter case, the marble has sufficient additional energy to ascend the wall after it has reached the lowest part of its descent. In either case, the marble will return to circling on the ledge. This essentially describes a stable equilibrium point for disruptive energy absorbed in an interaction.
PS: I should note that in this example I have disregarded time delays to convey momentum and relativistic effects, which were both used to develop the equation in the first place and are increasingly important closer to the nucleus.
PSS: It is a stable orbit. Please refer to Section 1.5 [ces May 6, 2002].
Q6: August 31, 2001: If, as you say, the size of the particle is caused by the charge and the size gives the particle its mass, why are the muon and electron different sizes? Why are protons and electrons different?, etc, etc.
A6: Allow me to use an analogy. Suppose we have two identical elastic balls (foam filled to prevent buckling) with a pump that fills them to a specified pressure. One ball is "filled" by the suction (negative pressure) side of the pump, while the second ball is filled by the positive pressure side. There are at least three physical characteristics to observe:
1) The direction of the pressure; 2) The magnitude of the pressure; 3) The temperature of the ball.
The direction of pressure is analogous to the sign of the charge. Positive pressure will result in a ball of larger size; negative pressure will produce a lesser size. The magnitude of the pressure produced by the pump is analogous to the magnitude of the charge. The temperature is analogous to the rest energy of the particle. Higher temperature (energy) will increase the size; cooler temperature will decrease it.
An analogy, however, does not make an argument. Understanding the underlying mechanisms of charge - how it is produced and why it exists, will either support or deny the validity of this concept.
Q5: July 9, 2001: I don't think a sub-atomic particle has a "Schwarzchild radius" similar to a black hole. There would be no surface to push against.
A5: Let me review what I mean when I say that an elemental massive particle has size. Let's consider a single electron and proton separated in space by one meter. We know a force exists between the particles due to their charge. If we wanted, we could map a series of orbits the electron could occupy (assuming it did not lose momentum). Each orbit would represent an equipotential surface (i.e. a sphere) where the electron's velocity is the same. Looking at this another way, each surface contour (i.e. orbit) represents a specific degree of time dilation relative to the proton. If we continue to draw these equipotential surfaces ever closer to the proton, we must encounter a point where the velocity reaches the speed of light - an event horizon - representing the "size" of the particle. Now this "event horizon" is not caused by gravity, but rather the charge within the particle.
But the question is "what would you push against?" Perhaps the answer is becoming obvious. It is the charge that interacts. When you stand on the floor, it is not due to atoms with electrons resembling ball bearings upon which you stand. Rather it is the charge of the electrons pushing away from each other - there is no "solid" surface needed.
Q4: May 15, 2001: Quantum Mechanics and, in particular, the Schrodinger Equation are excellent mathematical descriptions of reality. Why don't you agree with them?
A4: I will agree that the Schrodinger Equation is an extremely useful tool. I would like to frame my answer by positing several quotes from a very good book, "FRONTIERS Twentieth-Century Physics" by Steve Adams published by Taylor & Francis Ltd. Mr. Adams has done an excellent job presenting the conflict between Classic and Quantum physics.
The ideas of Quantum Mechanics yield:
"I am convinced that theoretical physics is actual philosophy." Max Born - Dedication page [if the philosophy is flawed, so is the physics - ces]
"... quantum theory undermined the very notions of reality and objectivity. ... reputable physicists publish papers outlining serious designs for teleportation and time machines." Steve Adams - Preface
"Born's view [of probability] is very different. Quantum mechanical probabilities do not arise from our ignorance of the microscopic configuration, they are fundamental." Steve Adams - page 21
"... it follows that quantum mechanics establishes the final failure of causality." Werner Heisenberg - page 35
"Bohr and Heisenberg removed the idea of a hidden mechanistic reality and placed indeterminism and complementarity at the heart of the new theory." Steve Adams - page 45
The ideas of Clasical physics:
"You believe in the god who plays dice, and I in complete law and order in a world which objectively exists..." excerpt from a letter from Einstein to Born - page 65
"Einstein believed that the physical world of atoms, electrons and photons exists independently of us and that the individual properties of particles are independent elements in an objectively real world." Steve Adams - page 76
So classical physics attempts to describe nature in ways that are consistent with reality (i.e. causal), whereas quantum mechanics attempts to "establish the final failure of causality". When physicists describe time machines that send matter into the past, they grossly violate energy conservation (mass is energy). But who's counting?
But, you might say, what about EPR (Einstein-Podolsky-Rosen) experiments that favor QM? To this I would suggest that the idea that photons exist as particles is flawed. Rather, I support the idea that momentum is transferred among electrons (and protons) via the electric field and, when time varying, sets up vast areas of constructive/destructive interference. Thus, when an electron is liberated from its orbit, sufficient constructive interference was the cause and a "photon" is said to be present. Remember, the only way we have of detecting "photons", is via electrons. Clearly, this approach allows measurement equipment to affect the outcome.
Q3: April 28, 2001: Here are two questions. First, do you consider fundamental and elemental to be synonymous? Second, you used the analogy of the Schwarzchild radius of the black hole to help describe the radius of a particle. Would you expect similar "orbits" around black holes?
A3: I used the word elemental to emphasize that the massive particle could no longer be subdivided any further into other massive particles and would require another means to describe its features. I would agree that the term fundamental is synonymous. I do not consider any particle that decays into other particles, fundamental or elemental. How can something, such as the neutron, which decays into other particles be elemental or fundamental? My analogy of the Schwarzchild radius was meant to draw attention to the similarity between the electrical and gravitational forces and that each affects space (i.e. space/time - see text) similarly. Indeed, equation 5 is the general form and could describe either a non-radiating electric or gravitational body. Thus, if equation 5 is correct, astronomers may well find small black holes orbiting a larger black hole without radiating gravitational waves.
Q2: March 27, 2001: You suggest that neutrinos are not only massive, but are not a single particle. What evidence is there to suggest this?
A2: What I am proposing (if I can review) is that all elemental massive particles occupy space and they do so because they have charge (read the text for further explanation). For a neutral "particle" to exist, it must have its charges cancel. Thus it must be composed of an EVEN number of unlike charged particles. I do not have evidence for or against this proposition. However, it should be noted that the neutron has been found to have a localized positive and negative charge.
Q1: March 18, 2001: Section 1.2 says that the neutron would be a "molecule" of other elemental massive particles - i.e. protons and electrons. This idea was proposed and discarded once it was found that the spin of a proton-electron neucleon was not consistent with experiment. Instead, the neutron is made of quarks, which are really the more elemental particles.
A1: This idea that a neutron is a proton-electron pair was indeed proposed and rejected on the grounds that you say. In the book, "Quarks and the Nature of the Universe - The Cosmic Onion" page 21 by Frank Close published by The American Institute of Physics, this notion is described. Briefly, the Nitrogen nucleus has integral spin, which means it must have an even number of particles with spin 1/2. If a neutron is composed of a proton-electron pair and 7 neutrons exist in the Nitrogen atom, then its nucleus would consist of 7 protons plus 7 proton-electron pairs - a total of 21 particles with spin 1/2 - an ODD number. However, the neutron decays into an electron, proton AND neutrino, and the neutrino being a lepton has spin 1/2. So if the neutron has a proton-electron-neutrino blend, it has multiple 1/2 spin. Thus, 7 protons plus 7 neutrons composed of proton-electron-neutrino blend is an EVEN number (i.e. 28) of spin 1/2 particles. Quarks may very well be an Elemental Massive Particle, however, at this time they have not been found to exist in a free state.